Ever wake up with a scratchy throat and think, “Great, my body’s army is on high alert again”? That’s your immune system doing its job—scouting for germs and swatting them down like an overcaffeinated bouncer at a club. But what keeps that same crew from roughing up your own tissues, sparking a full-on civil war inside? Enter the unsung heroes of immunology: a trio of scientists who’ve just snagged the 2025 Nobel Prize in Physiology or Medicine for cracking that code. Mary Brunkow, Fred Ramsdell, and Shimon Sakaguchi didn’t just map out our defenses—they spotlighted the chill-out squad that stops everything from spiraling into chaos.
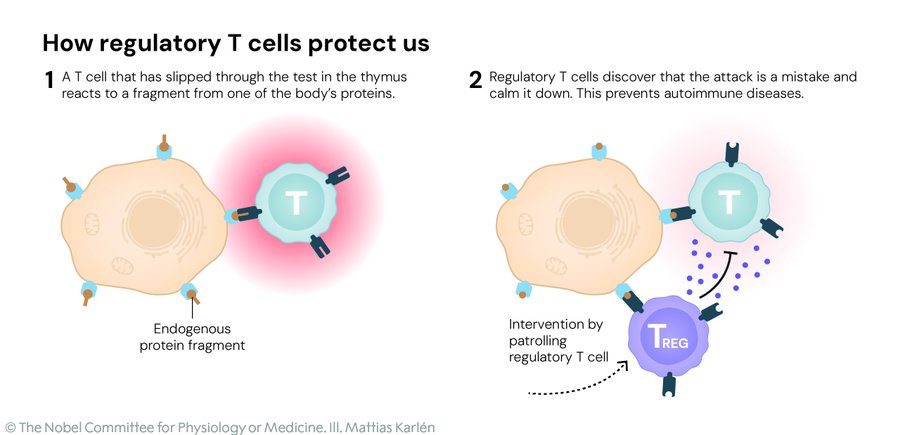
Picture the immune system as a rowdy neighborhood watch: eager killer T cells charge in to zap invaders, while helper T cells rally the troops. Without brakes, though, they’d start picking fights with innocent bystanders—your joints, your gut, even your brain—leading to the heartbreak of autoimmune diseases like rheumatoid arthritis or type 1 diabetes. The Nobel winners zeroed in on the regulators: special T cells, dubbed regulatory T cells or Tregs, that patrol the outskirts and whisper, “Stand down, folks—this is friendly territory.” It’s called peripheral immune tolerance, the body’s backup plan after the initial boot camp in the thymus weeds out the worst offenders.
The story kicks off in 1995 with Shimon Sakaguchi, a Japanese immunologist then at Japan’s Kyoto University, now a distinguished professor at Osaka University. While tinkering with mice stripped of their thymus glands—making them autoimmune powder kegs—he spotted a rare breed of CD4-positive T cells that could swoop in and restore the peace. These weren’t your average soldiers; they actively dialed back the aggression, preventing the body from turning on itself. It was a game-changer, resurrecting the long-forgotten idea of “suppressor cells” that had been dismissed as bunk just a decade earlier. Sakaguchi’s eureka moment? Proving the immune system had layers we barely understood, all happening right in the tissues where trouble brews.
Fast-forward to 2001, and across the Pacific, American researchers Mary Brunkow and Fred Ramsdell were knee-deep in a genetic mystery at the University of Washington. They were studying “scurfy” mice—poor little guys born with scaly skin, rampant inflammation, and a laundry list of organ failures from unchecked immune freak-outs. After years of gene-hunting drudgery, they nailed it: a glitch in a gene called Foxp3, the master switch for cranking out those regulatory T cells. Without it, the mice (and, crucially, kids with the human version of this mutation, a brutal syndrome called IPEX) were left defenseless against their own defenses. Brunkow, now a senior program manager at the Institute for Systems Biology in Seattle, and Ramsdell, a scientific advisor at Sonoma Biotherapeutics in San Francisco, essentially handed the field a genetic key to immune balance.
Sakaguchi looped it all together in 2003, linking his Tregs to Foxp3 and showing how this gene orchestrates their birth and behavior. Suddenly, scientists had a clear target: tweak these cells, and you could rewrite the rules of autoimmunity, cancer, and transplants. As Nobel Committee chair Olle Kämpe put it, their work has been “decisive for our understanding of how the immune system functions and why we do not all develop serious autoimmune diseases.” It’s the kind of breakthrough that feels like peering behind the curtain of our own biology—humbling, hopeful, and a tad terrifying in its implications.
So, what does this mean for the rest of us, beyond a pat on the back for three brilliant minds? Buckle up, because the ripple effects are hitting clinics hard. For the 50 million Americans wrestling with autoimmune woes, the dream is dialing up Tregs to hit the brakes on rogue attacks. Early trials are testing low-dose interleukin-2—a natural growth juice for these cells—to ease symptoms in folks with type 1 diabetes or graft-versus-host disease after stem cell transplants. Imagine infusing lab-grown Tregs tagged like GPS-guided missiles to zero in on inflamed joints in rheumatoid arthritis or the insulin-producing cells zapped in diabetes—it’s not sci-fi; it’s in the pipeline, with some patients already seeing glimmers of relief.
Flip the script to cancer, and it’s about stripping Tregs’ protective bubble around tumors. These cells often cozy up to malignancies, shushing the killer T cells that could otherwise mow them down—like a mob boss’s enforcers keeping the cops at bay. Drugs tweaking Foxp3 pathways or depleting Tregs locally are supercharging immunotherapies, potentially turning cold tumors hot and responsive for the millions facing breast, lung, or brain cancers.
And for transplants? That heart-wrenching wait for a match could get smoother. By boosting tolerance, Tregs might cut rejection risks, letting more patients thrive post-surgery without a lifetime of heavy immunosuppressants that leave them vulnerable to infections. It’s a quiet revolution, born from mouse labs and gene maps, but poised to touch lives in ways that tug at the heartstrings—restoring mobility to a mom with MS, extending playtime for a kid with lupus, or buying years for a cancer fighter.
In a world where our bodies can feel like fragile vessels adrift in a storm of unknowns, this Nobel feels like a lighthouse beam: proof that curiosity and grit can tame even the wildest tempests within. Here’s to the security guards we never knew we had—and the scientists who taught us to appreciate them.
This article is based on the official Nobel Prize announcements, including the press release and popular science summary from nobelprize.org.